
John Betts - Fine Minerals > Home Page> Educational Articles > Quick Test Method for Determining Specific Gravity of Mineral Specimens |

by John H. Betts, All Rights Reserved
UPDATE
I made the video below for the 2021 Rochester Mineralogical Symposium to explain the BETTS METHOD of measuring specific gravity. Watch it belolw:
THE ORIGINAL ARTICLE IS BELOW:
Specific gravity (sometimes referred to as Density) is a useful diagnostic attribute when trying to determine the mineral species of an unknown mineral specimen. But textbooks usually describe testing specific gravity using a laboratory balance scale that few of us have in our homes.
I have developed a quick technique, using a simple digital scale that determines specific gravity in only a few easy steps.

(For larger specimens I use a larger scale with a 5kg. maximum and a larger water container.)

1. Turn on power and the scale should "zero" itself.

2. Weigh the dry mineral specimen and record the weight.

3. Place the water-filled container (filled with water enough to submerge the specimen) on the scale and "zero" it out.
I size the cup of water to the size of the specimen. For small minerals, I use small cups. Because the paperclip displaces water, the small difference does not matter with a large cup of water, so zeroing out does not matter. But with a small cup you should zero the scale by suspending the paperclip in the water (not touching the sides or bottom) for best accuracy.

4. Suspend the specimen from the paperclip in the water, but not touching the bottom or sides and record the weight reading.


5. Divide the first (dry) weight by the second (suspended in water) weight and you get the specific gravity. The results for the crystal tested here was 2.627.
Done. Finished. Nothing else to do. Two measurements and some simple division.
Do not ask me how or why it works. The explanation will only confuse you. (SEE THE ADDENDUM AT THE BOTTOM IF YOU SIMPLY MUST KNOW WHY THIS WORKS.) All you need to know is that it works, it is fast, and it is easy and does not require any laboratory equipment. The only limitation is that the specimen must be homogenous - not a mixture of dissimilar minerals.
Now, what do you do with the specific gravity now that you know it? How do you find the mineral species that correspond to that number?
The easiest reference is the "Search for minerals by their physical properties" page at Mindat.com (http://www.mindat.org/advanced_search.php).
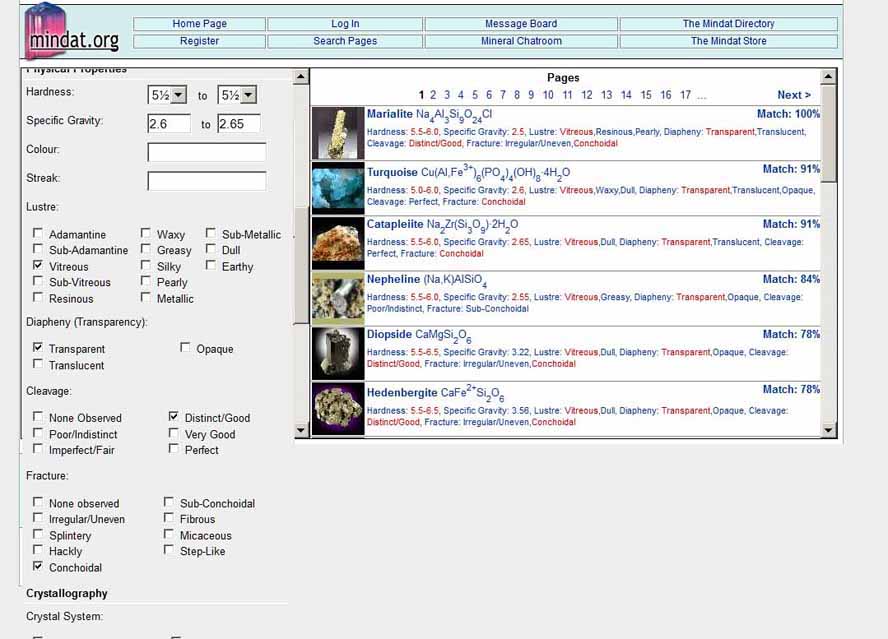
Above is a screen shot that shows I entered a range for specific gravity from 2.6 to 2.65 to allow for possible inaccuracies in the results.
I also entered values for other attributes that could be determined with simple tests or observations:
The search page shows on the right column the mineral species that match these characteristics and shows Marialite, a scapolite group mineral, closely corresponds to those attributes. This confirmed the identification that I suspected and eliminated gem albite-orthoclase which was a possible alternate.
Testing for specific gravity with this method is fast, easy, and accurate. Best of all it requires no expensive equipment. In the future, when you are confronted with an unknown mineral species, try this test and you will be amazed at how helpful the results are at narrowing the possibilities.
For those of you that want to know why this works, I will try to explain:
The normal method of determining specific gravity is to weigh the specimen dry (DW), then to suspend the specimen in water on a string, and to measure the weight pulling on the string (WW) (specimen weight suspended in water). Then you subtract WW from DW, and divide the difference into DW.
Example for a Specimen of lead:
Dry weight (DW): 705 grams
Wet Weight (WW): 642.6 grams
_____________________________
Difference (DW-WW): 62.4 grams
Specific gravity = 705 divided by 62.4 = 11.3 specific gravity
OK?
My method:
The weight of the specimen in water is pulling on the string less because a portion of the specimen weight is born by the water pushing down on the scale as you suspend the mineral.
In my method the same example (above) lead to results in these measurements:
Dry weight (DW): 705 grams
Weight of the water on the scale (zeroed out) with specimen suspended: 62.4 grams
Specific gravity = 705 divided by 62.4 = 11.3 specific gravity
Please support our sponsor
© John H. Betts - All Rights Reserved